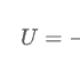Sections of the site
Editor's Choice:
- Razdolnoye (station) Historical chronicles of the settlement of razdolnoye prim krai
- Razdolnoye (station) Map of the village of razdolnoye, Primorsky Krai
- Where to meet the queen and bishop
- Jeep spare parts new and order from USA Vershinsky alexander nikolaevich sculptures
- "chita" can still be searched
- Which city was the capital before?
- Principality of Polotsk - Russian historical library Formation and demarcation of the principality
- Principality of Polotsk - Russian historical library Formation and demarcation of the principality
- History Kuznetsky Ostrog history
- Pontius Pilate - Fifth Procurator of Judea
Advertising
| What are X-rays - properties and applications of radiation. Lecture X-rays X-rays Sources Properties Applications |
|
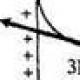
In 1895, the German physicist Roentgen, conducting experiments on the passage of current between two electrodes in a vacuum, discovered that a screen covered with a luminescent substance (barium salt) glows, although the discharge tube is covered with a black cardboard screen - this is how radiation was discovered that penetrates through opaque obstacles, called X-ray X-rays. It was found that X-rays, which are invisible to humans, are absorbed in opaque objects the stronger, the higher the atomic number (density) of the obstacle, so X-rays easily pass through the soft tissues of the human body, but are retained by the bones of the skeleton. High-power X-ray sources have been designed to show through metal parts and find internal defects in them. The German physicist Laue suggested that X-rays are the same electromagnetic radiation as the rays of visible light, but with a shorter wavelength and all the laws of optics are applicable to them, including the possible diffraction. In optics of visible light, diffraction at the elementary level can be represented as the reflection of light from a system of grooves - a diffraction grating, occurring only at certain angles, while the angle of reflection of the rays is related to the angle of incidence, the distance between the strokes of the diffraction grating and the wavelength of the incident radiation. For diffraction, the distance between the strokes must be approximately equal to the wavelength of the incident light. Laue suggested that X-rays have a wavelength close to the distance between individual atoms in crystals, i.e. the atoms in the crystal create an X-ray diffraction grating. X-rays directed at the surface of the crystal were reflected on the photographic plate, as predicted by theory. Any changes in the position of atoms affect the diffraction pattern, and by studying X-ray diffraction, one can find out the arrangement of atoms in a crystal and the change in this arrangement under any physical, chemical and mechanical effects on the crystal. Now X-ray analysis is used in many fields of science and technology, with its help they learned the arrangement of atoms in existing materials and created new materials with given structure and properties. Recent advances in this area (nanomaterials, amorphous metals, composite materials) create a field of activity for the next scientific generations. The appearance and properties of X-ray radiationThe X-ray source is an X-ray tube, which has two electrodes - a cathode and an anode. When the cathode is heated, electron emission occurs, the electrons emitted from the cathode are accelerated by the electric field and hit the surface of the anode. An X-ray tube is distinguished from a conventional radio tube (diode) mainly by a higher accelerating voltage (more than 1 kV). When an electron flies out of the cathode, the electric field makes it fly towards the anode, while its speed increases continuously, the electron carries a magnetic field, the strength of which increases with the electron's speed. Reaching the anode surface, the electron is sharply decelerated, and an electromagnetic pulse with wavelengths in a certain interval (bremsstrahlung) arises. The distribution of the radiation intensity over wavelengths depends on the material of the anode of the X-ray tube and the applied voltage; in this case, from the side of short waves, this curve starts from a certain threshold minimum wavelength, depending on the applied voltage. The collection of rays with all possible wavelengths forms a continuous spectrum, and the wavelength corresponding to the maximum intensity is 1.5 times the minimum wavelength. With increasing voltage, the X-ray spectrum changes dramatically due to the interaction of atoms with high-energy electrons and quanta of primary X-rays. An atom contains internal electron shells (energy levels), the number of which depends on the atomic number (denoted by the letters K, L, M, etc.) Electrons and primary X-rays knock electrons from one energy level to another. A metastable state arises, and a jump of electrons in the opposite direction is required for the transition to a stable state. This jump is accompanied by the release of a quantum of energy and the appearance of X-ray radiation. Unlike continuous-spectrum X-rays, this radiation has a very narrow wavelength range and high intensity (characteristic radiation) ( cm... rice.). The number of atoms that determine the intensity of the characteristic radiation is very large, for example, for an X-ray tube with a copper anode at a voltage of 1 kV and a current of 15 mA for 1 s, the characteristic radiation gives 10 14 –10 15 atoms. This value is calculated as the ratio of the total power of X-ray radiation to the energy of an X-ray quantum from the K-shell (K-series of characteristic X-ray radiation). In this case, the total power of X-ray radiation is only 0.1% of the consumed power, the rest is lost, mainly due to the transition to heat. Due to its high intensity and narrow wavelength range, characteristic X-ray radiation is the main type of radiation used in scientific research and process control. Simultaneously with the beams of the K-series, beams of the L and M-series are generated, having significantly longer wavelengths, but their use is limited. The K-series has two components with close wavelengths a and b, while the intensity of the b-component is 5 times less than that of a. In turn, the a-component is characterized by two very close wavelengths, the intensity of one of which is 2 times greater than the other. To obtain radiation with a single wavelength (monochromatic radiation), special methods have been developed that use the dependence of the absorption and diffraction of X-rays on the wavelength. An increase in the atomic number of an element is associated with a change in the characteristics of the electron shells, while the higher the atomic number of the X-ray tube anode material, the shorter the K-series wavelength. The most widely used tubes are with anodes of elements with atomic numbers from 24 to 42 (Cr, Fe, Co, Cu, Mo) and wavelengths from 2.29 to 0.712 A (0.229 - 0.712 nm). In addition to the X-ray tube, X-ray sources can be radioactive isotopes, some can directly emit X-rays, while others emit electrons and a-particles that generate X-rays when metal targets are bombarded. The intensity of X-ray radiation from radioactive sources is usually much less than that of an X-ray tube (with the exception of radioactive cobalt, which is used in flaw detection and gives radiation of a very short wavelength - g-radiation), they are small-sized and do not require electricity. Synchrotron X-ray radiation is obtained in electron accelerators, the wavelength of this radiation is much higher than that obtained in X-ray tubes (soft X-ray radiation), its intensity is several orders of magnitude higher than the radiation intensity of X-ray tubes. There are also natural sources of X-rays. Radioactive impurities are found in many minerals, X-rays from space objects, including stars, have been recorded. Interaction of X-rays with crystalsIn the X-ray study of materials with a crystal structure, interference patterns resulting from the scattering of X-rays by electrons belonging to the atoms of the crystal lattice are analyzed. Atoms are considered to be motionless, their thermal vibrations are not taken into account, and all electrons of the same atom are considered to be concentrated at one point - a site of the crystal lattice. To derive the basic equations of X-ray diffraction in a crystal, the interference of rays scattered by atoms located along a straight line in the crystal lattice is considered. A plane wave of monochromatic X-ray radiation is incident on these atoms at an angle whose cosine is equal to a 0. The laws of interference of rays scattered by atoms are similar to those existing for a diffraction grating that scatters light in the visible wavelength range. In order for the amplitudes of all oscillations to add up at a large distance from the atomic row, it is necessary and sufficient that the difference in the paths of the rays coming from each pair of neighboring atoms contain an integer number of wavelengths. At a distance between atoms a this condition has the form: a(a – a 0) = h l, where a is the cosine of the angle between the atomic row and the deflected ray, h - integer. In all directions that do not satisfy this equation, the rays do not propagate. Thus, the scattered rays form a system of coaxial cones, the common axis of which is the atomic row. The traces of the cones on the plane parallel to the atomic row are hyperbolas, and on the plane perpendicular to the row there are circles. When the rays are incident at a constant angle, polychromatic (white) radiation is decomposed into a spectrum of rays deflected at fixed angles. Thus, the atomic series is an X-ray spectrograph. Generalization to a two-dimensional (planar) atomic lattice, and then to a three-dimensional bulk (spatial) crystal lattice, gives two more similar equations, which include the angles of incidence and reflection of X-ray radiation and the distance between atoms in three directions. These equations are called Laue equations and are the basis of X-ray structural analysis. The amplitudes of the rays reflected from the parallel atomic planes add up, and since the number of atoms is very large, the reflected radiation can be detected experimentally. The reflection condition is described by the Wolfe - Bragg equation 2d sinq = nl, where d is the distance between adjacent atomic planes, q is the grazing angle between the direction of the incident beam and these planes in the crystal, l is the X-ray wavelength, n is an integer called the order of reflection. The angle q is the angle of incidence with respect to the atomic planes, which do not necessarily coincide in direction with the surface of the sample under study. Several methods of X-ray structural analysis have been developed, using both radiation with a continuous spectrum and monochromatic radiation. In this case, the object under study can be stationary or rotating, it can consist of one crystal (single crystal) or many (polycrystal), diffracted radiation can be recorded using a flat or cylindrical X-ray film or an X-ray detector moving around the circumference, however, in all cases during the experiment and the interpretation of the results uses the Wolfe - Bragg equation. X-ray analysis in science and technologyWith the discovery of X-ray diffraction, researchers have at their disposal a method that allows, without a microscope, to study the arrangement of individual atoms and changes in this arrangement under external influences. The main application of X-rays in basic science is structural analysis, i.e. establishing the spatial arrangement of individual atoms in a crystal. For this, single crystals are grown and X-ray analysis is carried out, studying both the location and the intensity of the reflections. Now the structures of not only metals, but also complex organic substances, in which the unit cells contain thousands of atoms, have been determined. In mineralogy, the structures of thousands of minerals have been determined by the method of retgenoanalysis and express methods have been developed for the analysis of mineral raw materials. Metals have a relatively simple crystal structure and the X-ray method allows one to study its changes during various technological treatments and create the physical foundations of new technologies. The position of the lines on the X-ray diffraction patterns determines the phase composition of the alloys, according to their width - the number, size and shape of crystals, according to the intensity distribution in the diffraction cone - the orientation of the crystals (texture). These techniques are used to study the processes during plastic deformation, including crushing of crystals, the occurrence of internal stresses and imperfections in the crystal structure (dislocations). When deformed materials are heated, stress relief and crystal growth (recrystallization) are studied. X-ray analysis of alloys determines the composition and concentration of solid solutions. When a solid solution appears, the interatomic distances change and, consequently, the distances between the atomic planes. These changes are small, therefore, special precision methods have been developed for measuring the periods of the crystal lattice with an accuracy two orders of magnitude higher than the measurement accuracy for conventional X-ray research methods. The combination of precision measurements of the crystal lattice periods and phase analysis makes it possible to plot the boundaries of the phase regions in the phase diagram. The X-ray method can also detect intermediate states between solid solutions and chemical compounds - ordered solid solutions in which impurity atoms are not randomly arranged, as in solid solutions, and at the same time, not with three-dimensional order, as in chemical compounds. There are additional lines on the X-ray diffraction patterns of ordered solid solutions; the interpretation of the X-ray diffraction patterns shows that impurity atoms occupy certain places in the crystal lattice, for example, at the vertices of a cube. When quenching an alloy that does not undergo phase transformations, a supersaturated solid solution may appear, and upon further heating or even holding at room temperature, the solid solution decomposes with the release of particles of a chemical compound. This is the aging effect and it manifests itself on radiographs as a change in the position and width of the lines. Aging research is especially important for non-ferrous alloys, for example, aging transforms a soft, hardened aluminum alloy into a tough structural material, duralumin. X-ray studies of heat treatment of steel are of the greatest technological importance. During quenching (rapid cooling) of steel, a diffusionless austenite - martensite phase transition occurs, which leads to a change in the structure from cubic to tetragonal, i.e. the unit cell takes the form of a rectangular prism. On radiographs, this manifests itself as an expansion of the lines and the separation of some lines into two. The reasons for this effect are not only a change in the crystal structure, but also the appearance of large internal stresses due to the thermodynamic nonequilibrium of the martensitic structure and abrupt cooling. During tempering (heating of hardened steel), the lines in the X-ray diffraction patterns narrow, this is due to the return to the equilibrium structure. In recent years, X-ray studies of the processing of materials with concentrated energy fluxes (laser beams, shock waves, neutrons, electron pulses) have acquired great importance; they demanded new techniques and gave new X-ray effects. For example, under the action of laser beams on metals, heating and cooling occur so rapidly that in the metal, upon cooling, crystals have time to grow only to a size of several unit cells (nanocrystals) or do not have time to appear at all. After cooling, such a metal looks like an ordinary metal, but does not give clear lines on the X-ray diffraction pattern, and the reflected X-rays are distributed over the entire range of grazing angles. After neutron irradiation, additional spots (diffuse maxima) appear on the X-ray diffraction patterns. Radioactive decay also causes specific X-ray effects associated with structural changes, as well as the fact that the sample under study itself becomes a source of X-ray radiation. X-ray radiation refers to electromagnetic waves with a wavelength of approximately 80 to 10 -5 nm. The longest-wavelength X-ray radiation is blocked by short-wavelength ultraviolet radiation, and the short-wavelength by long-wavelength γ-radiation. According to the excitation method, X-rays are divided into bremsstrahlung and characteristic. 31.1. X-RAY TUBE DEVICE. BRAKE X-RADIATION The most common X-ray source is the X-ray tube, which is a two-electrode vacuum unit (Figure 31.1). Heated cathode 1 emits electrons 4. Anode 2, often called the anti-cathode, has an inclined surface in order to direct the resulting X-ray radiation 3 at an angle to the tube axis. The anode is made of a good heat-conducting material to remove the heat generated by the electron impact. The surface of the anode is made of refractory materials with a large atomic number in the periodic table, for example, tungsten. In some cases, the anode is specially cooled with water or oil. For diagnostic tubes, the pinpoint of the X-ray source is important, which can be achieved by focusing electrons in one place of the anti-cathode. Therefore, constructively it is necessary to take into account two opposite problems: on the one hand, electrons must fall on one place of the anode, on the other hand, in order to prevent overheating, it is desirable to distribute electrons over different parts of the anode. One of the interesting technical solutions is the X-ray tube with a rotating anode (Fig. 31.2). As a result of deceleration of an electron (or other charged particle) by the electrostatic field of the atomic nucleus and atomic electrons of the substance of the anti-cathode, bremsstrahlung x-ray radiation. Its mechanism can be explained as follows. A moving electric charge is associated with a magnetic field, the induction of which depends on the speed of the electron. When braking, the magnetic induction and, in accordance with Maxwell's theory, an electromagnetic wave appears. When electrons are decelerated, only part of the energy goes to create an X-ray photon, the other part is spent on heating the anode. Since the relationship between these parts is random, then when a large number of electrons are decelerated, a continuous X-ray spectrum is formed. In this connection, bremsstrahlung is also called continuous. In fig. 31.3 shows the dependences of the X-ray flux on the wavelength λ (spectra) at different voltages in the X-ray tube: U 1< U 2 < U 3 . In each of the spectra, the shortest-wavelength bremsstrahlung is λ ηίη arises when the energy acquired by an electron in an accelerating field is completely converted into the energy of a photon:
Note that on the basis of (31.2) one of the most accurate methods of experimental determination of the Planck constant has been developed. Shortwave X-rays are usually more penetrating than longwave and are called tough and long-wave - soft. By increasing the voltage across the X-ray tube, the spectral composition of the radiation is changed, as can be seen from Fig. 31.3 and formulas (31.3), and increase the rigidity. If you increase the filament temperature of the cathode, then the emission of electrons and the current in the tube will increase. This will increase the number of X-ray photons emitted every second. Its spectral composition will not change. In fig. 31.4 shows the spectra of bremsstrahlung X-ray radiation at one voltage, but at different intensities of the cathode filament current: / h1< / н2 . The X-ray flux is calculated by the formula:
where U and I - X-ray tube voltage and current; Z- serial number of the anode substance atom; k- coefficient of proportionality. Spectra obtained from different anticathodes at the same U and I H are shown in Fig. 31.5.
31.2. CHARACTERISTIC X-RAY RADIATION. ATOMIC X-RAY SPECTRA By increasing the voltage across the X-ray tube, one can notice the appearance of a line-like line against the background of the continuous spectrum, which corresponds to characteristic X-ray radiation(fig.31.6). It arises due to the fact that accelerated electrons penetrate deep into the atom and knock out electrons from the inner layers. Electrons from the upper levels are transferred to free places (Fig. 31.7), as a result, photons of characteristic radiation are released. As can be seen from the figure, characteristic X-ray radiation consists of series K, L, M etc., the name of which served to designate the electronic layers. Since the radiation of the K-series frees up places in the higher layers, the lines of other series are also emitted at the same time. In contrast to optical spectra, the characteristic X-ray spectra of different atoms are of the same type. In fig. 31.8 shows the spectra of various elements. The uniformity of these spectra is due to the fact that the inner layers of different atoms are the same and differ only energetically, since the force effect from the side of the nucleus increases as the ordinal number of the element increases. This circumstance leads to the fact that the characteristic spectra shift towards higher frequencies with an increase in the nuclear charge. This pattern is seen from Fig. 31.8 and is known as Moseley's law:
where v - spectral line frequency; Z- the atomic number of the emitting element; A and V- permanent. There is another difference between optical and X-ray spectra. The characteristic X-ray spectrum of an atom does not depend on the chemical compound to which this atom is included. For example, the X-ray spectrum of the oxygen atom is the same for O, O 2 and H 2 O, while the optical spectra of these compounds are significantly different. This feature of the X-ray spectrum of the atom served as the basis for the name characteristic. Characteristic radiation always occurs when there is free space in the inner layers of an atom, regardless of the reason that caused it. For example, characteristic radiation accompanies one of the types of radioactive decay (see 32.1), which consists in the capture of an electron by the nucleus from the inner layer.
31.3. INTERACTION OF X-RAY RADIATION WITH SUBSTANCE Registration and use of X-ray radiation, as well as its effect on biological objects are determined by the primary processes of interaction of an X-ray photon with electrons of atoms and molecules of a substance. Depending on the energy ratio hv photon and ionization energy 1 A and there are three main processes. Coherent (classical) scattering Scattering of long-wavelength X-rays occurs mainly without changing the wavelength, and it is called coherent. It occurs if the photon energy is less than the ionization energy: hv< A and. Since in this case the energy of the X-ray photon and the atom does not change, then coherent scattering in itself does not cause a biological effect. However, when creating protection against X-ray radiation, one should take into account the possibility of changing the direction of the primary beam. This type of interaction is important for X-ray structural analysis (see 24.7). Incoherent scattering (Compton effect) In 1922 A.Kh. Compton, observing the scattering of hard X-rays, found a decrease in the penetrating power of the scattered beam in comparison with the incident one. This meant that the wavelength of the scattered X-ray radiation is greater than that of the incident one. The scattering of X-rays with a change in wavelength is called incoherent nym, and the phenomenon itself - the Compton effect. It occurs if the energy of the X-ray photon is greater than the ionization energy: hv> A and. This phenomenon is due to the fact that when interacting with an atom, the energy hv photon is spent on the formation of a new scattered X-ray photon with energy hv ", on the separation of an electron from an atom (ionization energy A and) and the transmission of kinetic energy to the electron E to: hv = hv "+ A and + E k.(31.6) 1 Here, the ionization energy is understood as the energy required to remove internal electrons outside the atom or molecule. Since in many cases hv>> And and the Compton effect occurs on free electrons, then we can write approximately: hv = hv "+ E K.(31.7) It is essential that in this phenomenon (Fig. 31.9), along with secondary X-ray radiation (energy hv photon), recoil electrons appear (kinetic energy E to electron). The atoms or molecules then become ions. Photo effect In the photoeffect, X-ray radiation is absorbed by the atom, as a result of which an electron is emitted, and the atom is ionized (photoionization). The three main interaction processes discussed above are primary, they lead to subsequent secondary, tertiary, etc. phenomena. For example, ionized atoms can emit a characteristic spectrum, excited atoms can become sources of visible light (X-ray luminescence), etc. In fig. 31.10 shows a diagram of possible processes that occur when X-ray radiation enters a substance. Several dozen processes similar to the one shown can occur before the energy of the X-ray photon converts into the energy of molecular-thermal motion. As a result, changes in the molecular composition of the substance will occur. The processes represented by the diagram in Fig. 31.10, underlie the phenomena observed when X-rays act on matter. Let's list some of them. X-ray luminescence- the glow of a number of substances under X-ray irradiation. This luminescence of platinum-cyanide barium allowed Roentgen to discover the rays. This phenomenon is used to create special luminous screens for the visual observation of X-rays, sometimes to enhance the effect of X-rays on a photographic plate. The chemical action of X-rays is known, for example the formation of hydrogen peroxide in water. A practically important example is the impact on a photographic plate, which makes it possible to fix such rays. The ionizing effect is manifested in an increase in electrical conductivity under the influence of X-rays. This property is used
in dosimetry to quantify the effects of this type of radiation. As a result of many processes, the primary X-ray beam is attenuated in accordance with law (29.3). Let's write it in the form: I = I 0 e- / ", (31.8) where μ is the linear attenuation coefficient. It can be represented as consisting of three terms corresponding to coherent scattering μ κ, incoherent μ ΗΚ, and photoelectric effect μ f: μ = μ k + μ hk + μ f. (31.9) The intensity of X-ray radiation is attenuated in proportion to the number of atoms of the substance through which this flow passes. If you compress the substance along the axis X, for example, in b times, increasing by b once its density, then
31.4. PHYSICAL BASIS OF APPLICATION OF X-RAY RADIATION IN MEDICINE One of the most important medical uses of X-rays is to scan internal organs for diagnostic purposes. (X-ray diagnostics). For diagnostics, photons with energies of the order of 60-120 keV are used. At this energy, the mass attenuation coefficient is mainly determined by the photoelectric effect. Its value is inversely proportional to the third power of the photon energy (proportional to λ 3), in which the high penetrating power of hard radiation is manifested, and is proportional to the third power of the atomic number of the absorbing substance:
A significant difference in the absorption of X-ray radiation by different tissues makes it possible to see images of the internal organs of the human body in the shadow projection. X-ray diagnostics are used in two ways: fluoroscopy - the image is viewed on an X-ray luminescent screen, radiography - the image is fixed on photographic film. If the examined organ and surrounding tissues attenuate X-ray radiation approximately equally, then special contrast agents are used. So, for example, filling the stomach and intestines with a mushy mass of barium sulfate, you can see their shadow image. The brightness of the image on the screen and the exposure time on the film depend on the intensity of the X-ray radiation. If it is used for diagnostics, then the intensity cannot be high, so as not to cause undesirable biological consequences. Therefore, there are a number of technical devices that improve the image at low intensities of X-ray radiation. An example of such a device is an image converter (see 27.8). In a mass examination of the population, a variant of radiography is widely used - fluorography, in which an image from a large X-ray luminescent screen is recorded on a sensitive small-format film. When shooting, a lens of high aperture is used, the finished images are examined with a special magnifier.
An interesting and promising option for radiography is a method called X-ray tomography, and its "machine version" - CT scan. Let's consider this question. A typical radiograph covers a large area of the body, with different organs and tissues obscuring each other. This can be avoided if periodically together (Fig. 31.11) in antiphase move the X-ray tube RT and film FP relative to the object About research. The body contains a number of inclusions opaque to X-rays, they are shown by circles in the figure. As you can see, X-rays at any position of the X-ray tube (1, 2 etc.) pass through cut the same point of the object, which is the center, relative to which the periodic movement is performed RT and FP. This point, or rather a small opaque inclusion, is shown by a dark circle. Its shadow image moves with FP, sequentially occupying positions 1, 2 etc. The rest of the inclusions in the body (bones, seals, etc.) are created on FP some general background, as X-rays are not constantly obscured by them. By changing the position of the swing center, a layer-by-layer X-ray image of the body can be obtained. Hence the name - tomography(layer-by-layer recording). It is possible, using a thin X-ray beam, a screen (instead of Фп), consisting of semiconductor detectors of ionizing radiation (see 32.5), and a computer, to process the shadow X-ray image during tomography. This modern version of tomography (computational or computed X-ray tomography) allows you to obtain layer-by-layer images of the body on the screen of a cathode-ray tube or on paper with details less than 2 mm with a difference in X-ray absorption of up to 0.1%. This allows, for example, to distinguish between gray and white matter of the brain and to see very small tumor formations. X-RAY RADIATION
If an electron hits a relatively heavy nucleus, then it is decelerated, and its kinetic energy is released in the form of an X-ray photon of approximately the same energy. If it flies past the nucleus, it will lose only part of its energy, and the rest will be transferred to other atoms that come across in its path. Each act of energy loss leads to the emission of a photon with some energy. A continuous X-ray spectrum appears, the upper limit of which corresponds to the energy of the fastest electron. This is the mechanism for the formation of a continuous spectrum, and the maximum energy (or minimum wavelength) that fixes the boundary of the continuous spectrum is proportional to the accelerating voltage, which determines the speed of the incident electrons. The spectral lines characterize the material of the bombarded target, and the continuous spectrum is determined by the energy of the electron beam and is practically independent of the target material. X-rays can be obtained not only by electron bombardment, but also by irradiating the target with X-rays from another source. In this case, however, most of the incident beam energy goes over into the characteristic X-ray spectrum, and a very small fraction of it falls on the continuous one. Obviously, the incident X-ray beam must contain photons, the energy of which is sufficient to excite the characteristic lines of the bombarded element. The high percentage of energy in the characteristic spectrum makes this method of X-ray excitation convenient for scientific research. X-ray tubes. To obtain X-ray radiation due to the interaction of electrons with matter, you need to have a source of electrons, means of accelerating them to high speeds and a target that can withstand electron bombardment and produce X-rays of the required intensity. The device that contains all of this is called an X-ray tube. Early researchers used "deep evacuated" tubes of the type of modern gas-discharge tubes. The vacuum in them was not very high. Gas discharge tubes contain a small amount of gas, and when a large potential difference is applied to the electrodes of the tube, the gas atoms are converted into positive and negative ions. The positive ones move to the negative electrode (cathode) and, falling on it, knock out electrons from it, and they, in turn, move to the positive electrode (anode) and, bombarding it, create a stream of X-ray photons. In the modern X-ray tube developed by Coolidge (Fig. 3), the source of electrons is a tungsten cathode heated to a high temperature. Electrons are accelerated to high speeds by the high potential difference between the anode (or anti-cathode) and the cathode. Since the electrons must reach the anode without colliding with the atoms, a very high vacuum is required, for which the tube must be well evacuated. This also reduces the probability of ionization of the remaining gas atoms and the resulting side currents.
The electrons are focused on the anode by a specially shaped electrode that surrounds the cathode. This electrode is called focusing and together with the cathode forms the "electron spotlight" of the tube. The electron bombarded anode must be made of a refractory material, since most of the kinetic energy of the bombarding electrons is converted into heat. In addition, it is desirable that the anode be made of a material with a high atomic number, since the X-ray yield increases with increasing atomic number. Tungsten is most often chosen as the anode material, the atomic number of which is 74. The design of X-ray tubes can be different depending on the conditions of use and the requirements.
To extend this approach to a three-dimensional crystal, it is only necessary to select the rows of atoms in two other directions in the crystal and solve the three equations thus obtained jointly for three crystal axes with periods a, b, and c. The other two equations are
l = 2 (d / n) sinq, where d is the distance between the planes with indices h, k and c (period), n = 1, 2, ... are integers (diffraction order), and q is the angle formed by incident beam (as well as diffracting) with the plane of the crystal in which diffraction occurs. Analyzing the equation of the Bragg - Wolfe law for a single crystal located in the path of a monochromatic X-ray beam, we can conclude that diffraction is not easy to observe, since the quantities l and q are fixed, and sinq DIFFRACTION ANALYSIS METHODS Laue method. The Laue method uses a continuous "white" X-ray spectrum, which is directed to a stationary single crystal. For a specific value of the period d, the wavelength value corresponding to the Bragg - Wolfe condition is automatically selected from the entire spectrum. The Laue patterns obtained in this way make it possible to judge the directions of the diffracted beams and, consequently, the orientations of the crystal planes, which also makes it possible to draw important conclusions regarding the symmetry, orientation of the crystal, and the presence of defects in it. In this case, however, information about the spatial period d is lost. In fig. 7 is an example of a Lauegram. The X-ray film was located on the side of the crystal opposite to that on which the X-ray beam from the source was incident.
Debye - Scherrer method (for polycrystalline samples). Unlike the previous method, monochromatic radiation (l = const) is used here, and the angle q is varied. This is achieved by using a polycrystalline sample consisting of numerous small crystallites of random orientation, among which there are those that satisfy the Bragg - Wolfe condition. The diffracted beams form cones, the axis of which is directed along the X-ray beam. A narrow strip of X-ray film in a cylindrical cassette is usually used for shooting, and the X-rays propagate in diameter through holes in the film. The Debyegram obtained in this way (Fig. 8) contains exact information about the period d, that is, about the structure of the crystal, but does not give the information that the Lauegram contains. Therefore, both methods complement each other. Let's consider some applications of the Debye - Scherrer method. Identification of chemical elements and compounds. The angle q determined from the Debyegram can be used to calculate the interplanar distance d characteristic of a given element or joint. Currently, many tables of d values have been compiled, which make it possible to identify not only one or another chemical element or compound, but also different phase states of the same substance, which does not always give chemical analysis. It is also possible in substitutional alloys to determine with high accuracy the content of the second component from the dependence of the period d on the concentration. Stress analysis. From the measured difference in interplanar distances for different directions in crystals, it is possible, knowing the modulus of elasticity of the material, to calculate with high accuracy the small stresses in it. Investigations of the preferred orientation in crystals. If small crystallites in a polycrystalline sample are not completely randomly oriented, then the rings on the Debyegram will have different intensities. In the presence of a sharply expressed predominant orientation, the intensity maxima are concentrated in separate spots in the image, which becomes similar to the image for a single crystal. For example, during deep cold rolling, a metal sheet acquires a texture - a pronounced orientation of crystallites. The Debyegram can be used to judge the nature of the cold working of the material. Investigation of grain sizes. If the grain size of the polycrystal is more than 10-3 cm, then the lines on the Debyegram will consist of separate spots, since in this case the number of crystallites is insufficient to cover the entire range of values of the angles q. If the crystallite size is less than 10-5 cm, then the diffraction lines become wider. Their width is inversely proportional to the size of the crystallites. The broadening occurs for the same reason that, with a decrease in the number of slits, the resolution of the diffraction grating decreases. X-ray radiation makes it possible to determine grain sizes in the range of 10-7-10-6 cm. Methods for single crystals. In order for diffraction by a crystal to give information not only about the spatial period, but also about the orientation of each set of diffracting planes, methods of a rotating single crystal are used. A monochromatic X-ray beam is incident on the crystal. The crystal rotates around the main axis for which the Laue equations are satisfied. This changes the angle q included in the Bragg - Wolfe formula. The diffraction maxima are located at the intersection of the Laue diffraction cones with the cylindrical surface of the film (Fig. 9). The result is a diffraction pattern of the type shown in Fig. 10. However, complications are possible due to the overlap of different diffraction orders at one point. The method can be significantly improved if, simultaneously with the rotation of the crystal, the film is also moved in a certain way.
Studies of liquids and gases. It is known that liquids, gases and amorphous bodies do not have the correct crystal structure. But even here there is a chemical bond between atoms in molecules, due to which the distance between them remains almost constant, although the molecules themselves in space are oriented randomly. Such materials also give a diffraction pattern with a relatively small number of diffuse maxima. The processing of such a picture by modern methods makes it possible to obtain information on the structure of even such non-crystalline materials. SPECTROCHEMICAL X-RAY ANALYSIS Already a few years after the discovery of X-rays, C. Barclay (1877-1944) discovered that when a substance is exposed to a high-energy X-ray radiation stream, a secondary fluorescent X-ray radiation is generated, which is characteristic of the element under study. Soon after this, G. Moseley, in a series of his experiments, measured the wavelengths of the primary characteristic X-ray radiation received by electron bombardment of various elements, and derived the relationship between the wavelength and atomic number. These experiments, as well as Bragg's invention of the X-ray spectrometer, laid the foundation for spectrochemical X-ray analysis. The possibilities of X-rays for chemical analysis were immediately recognized. Spectrographs were created with registration on a photographic plate, in which the sample under study served as the anode of an X-ray tube. Unfortunately, this technique turned out to be very laborious, and therefore was used only when the usual methods of chemical analysis were inapplicable. An outstanding example of innovative research in the field of analytical X-ray spectroscopy was the discovery in 1923 by G. Hevesy and D. Koster of a new element - hafnium. The development of high-power X-ray tubes for radiography and sensitive detectors for radiochemical measurements during World War II largely contributed to the rapid growth of X-ray spectrography in the following years. This method has become widespread due to the speed, convenience, non-destructive nature of the analysis and the possibility of full or partial automation. It is applicable in the problems of quantitative and qualitative analysis of all elements with an atomic number greater than 11 (sodium). Although X-ray spectrochemical analysis is usually used to determine the most important components in a sample (0.1-100%), in some cases it is suitable for concentrations of 0.005% or even lower. X-ray spectrometer. A modern X-ray spectrometer consists of three main systems (Fig. 11): an excitation system, i.e. an X-ray tube with an anode made of tungsten or other refractory material and a power supply unit; analysis systems, i.e. an analyzer crystal with two multi-slit collimators, as well as a spectrogoniometer for precise alignment; and recording systems with a Geiger counter or proportional or scintillation counter, as well as a rectifier, amplifier, counters and recorder or other recording device.
X-ray fluorescence analysis. The sample to be analyzed is located in the path of the exciting X-ray radiation. The area of the sample to be examined is usually distinguished by a mask with a hole of the required diameter, and the radiation passes through a collimator that forms a parallel beam. Behind the analyzer crystal, a slit collimator releases diffracted radiation for the detector. Usually, the maximum angle q is limited to values of 80-85 °, so that only the X-ray radiation whose wavelength l is related to the interplanar distance d by the inequality l X-ray microanalysis. The above-described flat crystal analyzer spectrometer can be adapted for microanalysis. This is achieved by narrowing either the primary X-ray beam or the secondary beam emitted from the sample. However, a decrease in the effective size of the sample or the radiation aperture leads to a decrease in the intensity of the recorded diffracted radiation. Improvement of this method can be achieved by using a bent crystal spectrometer, which allows recording a cone of diverging radiation, and not just radiation parallel to the collimator axis. Particles smaller than 25 µm can be identified with such a spectrometer. An even greater reduction in the size of the analyzed sample is achieved in the electron probe X-ray microanalyzer invented by R. Kasten. Here, the characteristic X-ray radiation of the sample is excited by a sharply focused electron beam, which is then analyzed by a bent crystal spectrometer. Using such a device, it is possible to detect amounts of a substance of the order of 10-14 g in a sample with a diameter of 1 μm. Installations with electron-beam scanning of the sample were also developed, with the help of which it is possible to obtain a two-dimensional picture of the distribution over the sample of the element, for the characteristic radiation of which the spectrometer is tuned. MEDICAL X-RAY DIAGNOSTICS The development of X-ray technology has made it possible to significantly reduce the exposure time and improve the quality of images, allowing even soft tissues to be studied. Fluorography. This diagnostic method consists in photographing a shadow image from a translucent screen. The patient is placed between the X-ray source and a flat screen made of a phosphor (usually cesium iodide), which glows when exposed to the X-ray radiation. Biological tissues of varying degrees of density create x-ray shadows with varying degrees of intensity. The radiologist examines the shadow image on the fluorescent screen and makes a diagnosis. In the past, a radiologist relied on vision to analyze images. Now there are various systems that amplify the image, display it on a television screen or record data in the computer's memory. Radiography. Recording an X-ray image directly on photographic film is called radiography. In this case, the organ under study is located between the X-ray source and the photographic film, which records information about the state of the organ at a given time. Repeated radiography makes it possible to judge its further evolution. Radiography allows very accurate examination of the integrity of bone tissue, which consists mainly of calcium and is opaque to X-ray radiation, as well as muscle tissue rupture. With its help, better than a stethoscope or listening, the condition of the lungs is analyzed in case of inflammation, tuberculosis or the presence of fluid. With the help of radiography, the size and shape of the heart, as well as the dynamics of its changes in patients with heart disease, are determined. Contrast agents. Parts of the body and cavities of individual organs that are transparent for X-ray radiation become visible if they are filled with a contrast agent that is harmless to the body, but allows visualizing the shape of internal organs and checking their functioning. The patient either takes contrast agents by mouth (such as barium salts when examining the gastrointestinal tract), or they are injected intravenously (such as iodine-containing solutions when examining the kidneys and urinary tract). In recent years, however, these methods have been superseded by diagnostic methods based on the use of radioactive atoms and ultrasound. CT scan. In the 1970s, a new method of X-ray diagnostics was developed, based on a complete survey of the body or its parts. Images of thin layers ("slices") are processed by a computer, and the final image is displayed on the monitor screen. This method is called computed x-ray tomography. It is widely used in modern medicine to diagnose infiltrates, tumors and other brain disorders, as well as to diagnose soft tissue diseases inside the body. This technique does not require the introduction of foreign contrast agents and therefore is faster and more effective than traditional techniques. BIOLOGICAL EFFECTS OF X-RAY RADIATION The harmful biological effect of X-rays was discovered shortly after its discovery by Roentgen. It turned out that the new radiation can cause something like a severe sunburn (erythema), accompanied, however, by deeper and more persistent skin damage. The ulcers that appeared often turned into cancer. In many cases, fingers or hands had to be amputated. There were also fatalities. It has been found that skin damage can be avoided by reducing the time and dose of radiation by using shielding (eg lead) and remote controls. But gradually other, more long-term effects of X-ray exposure came to light, which were then confirmed and studied in experimental animals. The effects due to the action of X-rays, as well as other ionizing radiation (such as gamma radiation emitted by radioactive materials) include: 1) temporary changes in the composition of the blood after relatively little excess exposure; 2) irreversible changes in blood composition (hemolytic anemia) after prolonged excessive exposure; 3) an increase in the incidence of cancer (including leukemia); 4) faster aging and early death; 5) the occurrence of cataracts. In addition, biological experiments on mice, rabbits and flies (fruit flies) have shown that even small doses of systematic irradiation of large populations, due to an increase in the rate of mutation, lead to harmful genetic effects. Most geneticists recognize the applicability of this data to the human body. As for the biological effect of X-ray radiation on the human body, it is determined by the level of the radiation dose, as well as by which organ of the body was exposed to radiation. For example, blood diseases are caused by irradiation of the blood-forming organs, mainly the bone marrow, and genetic consequences are caused by irradiation of the genitals, which can also lead to sterility. The accumulation of knowledge about the effects of X-ray radiation on the human body has led to the development of national and international standards for permissible radiation doses, published in various reference publications. In addition to X-ray radiation, which is purposefully used by humans, there is also the so-called scattered, spurious radiation that occurs for various reasons, for example, due to scattering due to imperfection of the lead protective shield, which does not completely absorb this radiation. In addition, many electrical devices that are not designed to produce x-rays nevertheless generate x-rays as a by-product. Such devices include electron microscopes, high-voltage rectifier lamps (kenotrons), and also picture tubes of obsolete color televisions. The production of modern color picture tubes in many countries is now under government control. DANGEROUS FACTORS OF X-RAY RADIATION The types and degree of danger of X-ray exposure for people depend on the contingent of persons exposed to radiation. Professionals working with X-ray equipment. This category covers radiologists, dentists, as well as scientific and technical workers and personnel who maintain and use X-ray equipment. Effective measures are being taken to reduce the level of radiation they have to deal with. Patients. There are no strict criteria here, and the safe level of radiation that patients receive during treatment is determined by the attending physicians. Doctors are not advised to unnecessarily expose patients to x-rays. Particular care should be taken when examining pregnant women and children. In this case, special measures are taken. Control methods. There are three aspects to this: 1) the availability of adequate equipment, 2) monitoring compliance with safety regulations, 3) the correct use of equipment. X-ray examinations should only expose the desired area to radiation, whether for dental examinations or lung examinations. Note that immediately after turning off the X-ray apparatus, both primary and secondary radiation disappears; there is also no residual radiation, which is not always known even by those who are directly related to it in their work. see also ATOM BUILDING; Modern medical diagnostics and treatment of some diseases cannot be imagined without devices that use the properties of X-ray radiation. The discovery of X-rays happened more than 100 years ago, but even now work continues on the creation of new techniques and devices that make it possible to minimize the negative effect of radiation on the human body. Who and how discovered X-raysUnder natural conditions, the flux of X-rays is rare and is emitted only by some radioactive isotopes. X-rays or X-rays were discovered only in 1895 by the German scientist Wilhelm Röntgen. This discovery happened by chance, during an experiment to study the behavior of light rays in conditions approaching a vacuum. The experiment involved a cathode gas-discharge tube with a reduced pressure and a fluorescent screen, which started glowing every time the tube started to work. Intrigued by the strange effect, Roentgen conducted a series of studies showing that the resulting radiation invisible to the eye can penetrate various obstacles: paper, wood, glass, some metals, and even through the human body. Despite the lack of understanding of the very nature of what is happening, whether such a phenomenon is caused by the generation of a stream of unknown particles or waves, the following pattern was noted - radiation easily passes through the soft tissues of the body, and much heavier through hard living tissues and inanimate substances.
In 1901, a fruitful collaboration began between three scientists who became the founding fathers of radiology and radiology.
X-ray propertiesX-rays are part of the overall spectrum of electromagnetic radiation. The wavelength is located between gamma and ultraviolet rays. X-rays are characterized by all the usual wave properties:
For the artificial generation of a flux of X-rays, special devices are used - X-ray tubes. X-rays arise from the contact of fast electrons of tungsten with substances evaporating from a hot anode. Against the background of the interaction, electromagnetic waves of small length appear, which are in the spectrum from 100 to 0.01 nm and in the energy range of 100 to 0.1 MeV. If the wavelength of the rays is less than 0.2 nm, it is hard radiation, if the wavelength is greater than the specified value, they are called soft X-rays.
X-ray radiation - bremsstrahlung and characteristicX-radiation is a superposition of two types of rays - bremsstrahlung and characteristic rays. They are generated in the tube at the same time. Therefore, the X-ray irradiation and the characteristic of each specific X-ray tube - the spectrum of its radiation, depends on these indicators, and represents their overlap. Braking or continuous X-rays are the result of the braking of electrons evaporated from the tungsten coil. Characteristic or linear X-ray beams are formed at the moment of rearrangement of the atoms of the X-ray tube anode. The wavelength of the characteristic rays directly depends on the atomic number of the chemical element used to make the tube anode. The listed properties of X-rays make it possible to apply them in practice:
Principles of obtaining an x-ray imageThe properties of X-rays on which the imaging is based is the ability to either decompose or cause some substances to glow. X-ray irradiation causes a fluorescent glow in cadmium and zinc sulfides - green, and in calcium tungstate - blue. This property is used in the technique of medical X-ray transillumination, and also increases the functionality of X-ray screens. The photochemical effect of X-rays on light-sensitive silver halide materials (light exposure) allows for diagnostics - making X-ray images. This property is also used when measuring the value of the total dose that laboratory technicians receive in X-ray rooms. Body-worn dosimeters have special sensitive tapes and indicators. The ionizing effect of X-rays makes it possible to determine the qualitative characteristics of the obtained X-rays.
Areas where X-rays are usedThe use of X-rays is acceptable in the following industries:
The main application of X-rays is in the medical field. Today, the section of medical radiology includes: radiodiagnostics, radiotherapy (X-ray therapy), radiosurgery. Medical universities graduate narrow-profile specialists - radiologists.
X-Radiation - harm and benefit, effect on the bodyThe high penetrating power and ionizing effect of X-rays can cause a change in the structure of the cell's DNA, therefore, it is dangerous for humans. The damage from X-rays is directly proportional to the radiation dose received. Different organs respond to radiation to varying degrees. The most susceptible are:
Uncontrolled use of X-ray radiation can cause reversible and irreversible pathologies. Consequences of X-ray exposure:
The use of X-rays in medicine is permissible not only for diagnostic (traumatology, dentistry), but also for therapeutic purposes:
Methods for diagnosing pathologies using X-raysRadio diagnostics includes the following techniques:
RadiotherapyX-ray therapy is a topical treatment. Most often, the method is used to destroy cancer cells. Since the effect of exposure is comparable to surgical removal, this method of treatment is often called radiosurgery. Today, x-ray treatment is carried out in the following ways:
Safe X-ray exposure rateThis indicator of the permissible annual exposure rate has its own name - a genetically significant equivalent dose (GZD). This indicator has no clear quantitative values.
Today, the following averaged standards for the HDM are in force:
X-ray radiation does not need to be removed from the body, and is dangerous only in case of intense and prolonged exposure. Modern medical equipment uses low-energy radiation of short duration, so its use is considered relatively harmless. X-ray radiation occurs when electrons moving at high speeds interact with matter. When electrons collide with atoms of any substance, they quickly lose their kinetic energy. In this case, most of it turns into heat, and a small fraction, usually less than 1%, is converted into X-ray energy. This energy is released in the form of quanta - particles called photons that have energy but whose rest mass is zero. X-ray photons differ in their energy, which is inversely proportional to their wavelength. The conventional method of producing X-rays produces a wide range of wavelengths called the X-ray spectrum. The spectrum contains pronounced components, as shown in Fig. 1. Rice. 1. REGULAR X-RAY SPECTRUM consists of a continuous spectrum (continuum) and characteristic lines (sharp peaks). Lines Кia and Кib appear due to interactions of accelerated electrons with electrons of the inner K-shell. The broad "continuum" is called continuous spectrum or white radiation. The sharp peaks superimposed on it are called characteristic X-ray emission lines. Although the entire spectrum is the result of collisions of electrons with matter, the mechanisms for the emergence of its wide part and lines are different. The substance consists of a large number of atoms, each of which has a nucleus surrounded by electron shells, and each electron in the shell of an atom of a given element occupies a certain discrete energy level. Usually these shells, or energy levels, are denoted by the symbols K, L, M, etc., starting from the shell closest to the core. When an incident electron with a sufficiently high energy collides with one of the electrons bound to the atom, it knocks that electron out of its shell. The empty place is occupied by another electron from the shell, which corresponds to a large energy. This latter gives up excess energy by emitting an X-ray photon. Since the electrons of the shells have discrete energy values, the emerging X-ray photons also have a discrete spectrum. This corresponds to sharp peaks for certain wavelengths, the specific values of which depend on the target element. The characteristic lines form the K-, L- and M-series, depending on which shell (K, L or M) the electron was removed from. The relationship between the wavelength of X-rays and the atomic number is called Moseley's law (Fig. 2).  Rice. 2. The WAVE LENGTH of the CHARACTERISTIC X-RAY RADIATION emitted by chemical elements depends on the atomic number of the element. The curve corresponds to Moseley's law: the higher the atomic number of an element, the shorter the wavelength of the characteristic line. If an electron hits a relatively heavy nucleus, then it is decelerated, and its kinetic energy is released in the form of an X-ray photon of approximately the same energy. If it flies past the nucleus, it will lose only part of its energy, and the rest will be transferred to other atoms that come across in its path. Each act of energy loss leads to the emission of a photon with some energy. A continuous X-ray spectrum appears, the upper limit of which corresponds to the energy of the fastest electron. This is the mechanism for the formation of a continuous spectrum, and the maximum energy (or minimum wavelength) that fixes the boundary of the continuous spectrum is proportional to the accelerating voltage, which determines the speed of the incident electrons. The spectral lines characterize the material of the bombarded target, and the continuous spectrum is determined by the energy of the electron beam and is practically independent of the target material. X-rays can be obtained not only by electron bombardment, but also by irradiating the target with X-rays from another source. In this case, however, most of the incident beam energy goes over into the characteristic X-ray spectrum, and a very small fraction of it falls on the continuous one. Obviously, the incident X-ray beam must contain photons, the energy of which is sufficient to excite the characteristic lines of the bombarded element. The high percentage of energy in the characteristic spectrum makes this method of X-ray excitation convenient for scientific research. X-ray tubes. To obtain X-ray radiation due to the interaction of electrons with matter, you need to have a source of electrons, means of accelerating them to high speeds and a target that can withstand electron bombardment and produce X-rays of the required intensity. The device that contains all of this is called an X-ray tube. Early researchers used "deeply evacuated" tubes of the type of modern gas-discharge tubes. The vacuum in them was not very high. Gas discharge tubes contain a small amount of gas, and when a large potential difference is applied to the electrodes of the tube, the gas atoms are converted into positive and negative ions. The positive ones move to the negative electrode (cathode) and, falling on it, knock out electrons from it, and they, in turn, move to the positive electrode (anode) and, bombarding it, create a stream of X-ray photons. In the modern X-ray tube developed by Coolidge (Fig. 3), the source of electrons is a tungsten cathode heated to a high temperature. Electrons are accelerated to high speeds by the high potential difference between the anode (or anti-cathode) and the cathode. Since the electrons must reach the anode without colliding with the atoms, a very high vacuum is required, for which the tube must be well evacuated. This also reduces the probability of ionization of the remaining gas atoms and the resulting side currents.  Rice. 3. COULIDGE X-RAY TUBE. When bombarded with electrons, the tungsten anti-cathode emits characteristic X-rays. The cross section of the X-ray beam is smaller than the actual irradiated area. 1 - electron beam; 2 - cathode with focusing electrode; 3 - glass shell (tube); 4 - tungsten target (anti-cathode); 5 - filament of the cathode; 6 - actually irradiated area; 7 - effective focal spot; 8 - copper anode; 9 - window; 10 - scattered X-ray radiation. The electrons are focused on the anode by a specially shaped electrode that surrounds the cathode. This electrode is called focusing and together with the cathode forms the "electron spotlight" of the tube. The electron bombarded anode must be made of a refractory material, since most of the kinetic energy of the bombarding electrons is converted into heat. In addition, it is desirable that the anode be made of a material with a high atomic number, since the X-ray yield increases with increasing atomic number. Tungsten is most often chosen as the anode material, the atomic number of which is 74. The design of X-ray tubes can vary depending on the application and requirements. |
New
- Sulfuric acid: chemical properties, characteristics, production of sulfuric acid in production
- The main types of human activities
- The confrontation of civilizations in the cultural heritage of the North-West of Russia: the Novgorod period
- Uranium half-life: basic characteristics and applications Radioactive uranium 235 92
- Transactional analysis as an effective therapeutic technique in narcology
- Stalin's atomic legacy What is uranium 235
- Methods of pedagogical activity
- Local, World Time, Standard Time and Daylight Saving Time What is Daylight Saving Time for?
- Newton's classical theory of gravitation
- Andrey Geim, modern scientist physicist: biography, scientific achievements, awards and prizes