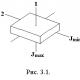
Sections of the site
Editor's Choice:
- Crimping the content of international environmental legal protection
- Kamenka (Penza region) to which federal district belongs to the city of Kamenka
- Russian principalities and land in the XII - XIII centuries
- Charles de Gaulle: Biography and Interesting Facts From the life of Charles de Gaulle Right or left
- Where does the water disappears in the kettle?
- The degree of oxidation (oxidative number, oxidation state) is H2O2 the degree of oxidation
- History of biological research
- Hydrogen Communication All Examples
- Barbaric Encyclopedia: Commod, Emperor Lucius Ely Aureli Commoda Mark Azeri Son
- History of creation and development
Advertising
| How the properties of elements are changed in a group. Patterns of changes in the chemical properties of elements. Characteristics of elements. Changing the properties of elements within the period |
|
Answer: Patterns of changes in the chemical properties of elements and their compounds in periods and groupsWe list the patterns of changes in properties that manifest themselves within periods: - metal properties decrease; - non-metallic properties are enhanced; - the degree of oxidation of elements in higher oxides increases from $ + $ 1 to $ + $ 7 ($ + $ 8 for $ os $ and $ ru $); - the degree of oxidation of elements in volatile hydrogen compounds increases from $ -4 $ to $ -1 $; - oxides from the main through amphoteric are replaced by acid oxides; - Hydroxides from alkali through amphoteric replaced by acids. D. I. Mendeleev at $ 1869 G. concluded - formulated a periodic law, which sounds like this: Properties chemical elements and substances they formed are in periodic dependence on the relative atomic masses of the elements. Sourcing chemical elements based on their relative atomic masses, Mendeleev paid great attention to the properties of elements and substances formed by them, distributing elements with similar properties to vertical columns - groups. Sometimes, in violation of the law identified by him, Mendeleev put heavier elements with less values \u200b\u200bof relative atomic masses. For example, he recorded a cobalt in his table in front of nickel, tellurium in front of iodine, and when inert (noble) gases were opened, - argon before Kali. Such a procedure for the location of Mendeleev considered it necessary because otherwise these elements would fall into groups of unrequisite with them according to the properties of elements, in particular, the Potassium alkaline metal would fall into the group of inert gases, and the inert gas argon - to the group alkali metals. D. I. Mendeleev could not explain these exceptions from the general rule, could not explain the reason for the reason for the periodicity of the properties of the elements and the substances they formed. However, he foresaw that this reason lies in the complex structure of the atom, internal structure which at that time was not studied. In accordance with modern ideas about the structure of the atom, the basis of the classification of chemical elements are the charges of their atomic nuclei, and the modern formulation of the periodic law is: The properties of chemical elements and substances they formed are in periodic dependence on the charges of their atomic nuclei. The frequency in the change in the properties of the elements is explained by periodic repeatability in the structure of the external energy levels of their atoms. It is the number of energy levels, total number electrons arranged on them and the number of electrons at the external level reflect the symbolism adopted in the periodic system, i.e. Reveal physical meaning Period numbers, group numbers and sequence element number. The structure of the atom allows you to explain the causes of changes in the metal and non-metallic properties of elements in periods and groups. Periodic law and periodic system of chemical elements D. I. Mendeleev summarize the information on the chemical elements and the substances they formed and explain the frequency in changing their properties and the cause of the similarity of the properties of the elements of the same group. These two most important values \u200b\u200bof the periodic law and the periodic system complements another one, which is possible to predict, i.e. Predict, describe properties and indicate ways to open new chemical elements. The overall characteristics of metals of the main subgroups I ± III groups due to their position in the periodic system of chemical elements by D. I. Mendeleev and the characteristics of the structure of their atomsChemical elements - metalsMost chemical elements are related to metals - $ 92 $ from $ 114 $ known elements. All metals, except mercury, in the usual condition - solids and have a number of common properties. Metals. - these are damp, plastic, drig, having a metal shine and are able to carry out heat and electric current. The atoms of metal elements give electrons of the external (and some and the pretended) electronic layer, turning into positive ions. This property of metals atoms, as you know, is determined by the fact that they have relatively large radii and a small number of electrons (mainly from $ 1 $ to $ 3 $ on the outer layer). An exception is only $ 6 $ metals: Germany, tin atoms, lead on the outer layer have a $ 4 $ electron, antimony and bismuth atoms - $ 5 $, half aim atoms - $ 6 $. For metals atoms, small electronegability values \u200b\u200bare characterized (from $ 0.7 $ to $ 1.9 $) and exclusively rehabilitation properties, i.e. The ability to give electrons. You already know that in the periodic system of chemical elements D. I. Mendeleev Metals are below diagonal Bor - Astat, as well as above it, in side subgroups. In the periods and main subgroups, the regularities in the change in metallic, and therefore the restoration properties of the atoms of the elements are valid. Chemical elements located near the Boron diagonal - Astat ($ BE, \u200b\u200bAl, Ti, Ge, Nb, SB $), have dual properties: in some of its compounds behave like metals, in others there are properties of non-metals. In adverse subgroups, the restoration properties of metals with an increase in the sequence number are most often reduced. This can be explained by the fact that the strength of the supply of valence electrons with the nucleus at atoms of these metals is more affected by the magnitude of the kernel, and not the radius of the atom. The magnitude of the charge of the kernel increases significantly, the attraction of electrons to the kernel is enhanced. The radius of the atom is also increasing, but not so much as the main subgroups. Simple substances formed by chemical elements - metals, and complex metal-containing substances play a crucial role in the mineral and organic "life" of the Earth. It suffices to recall that atoms (ions) of metal elements are part of compounds that determine the metabolism in the human body, animals. For example, $ 76 $ elements were found in the blood of a person, of which only $ 14 $ are not metals. In the human body, some elements are metals (calcium, potassium, sodium, magnesium) are present in large quantities, i.e. are macroelements. And such metals, like chrome, manganese, iron, cobalt, copper, zinc, molybdenum are present in small quantities, i.e. this is trace elements. Features of the structure of metals of the main subgroups of the I-III groups. Alkali metals - These are metals of the main subgroup I group. Their atoms at the external energy level have one electron. Alkali metals - strong reducing agents. Their rehabilitation and chemical activity increase with an increase in the sequence number of the element (i.e. from top to bottom in Periodic table). All of them possess electronic conductivity. The strength of communication between alkali metal atoms is reduced with an increase in the sequence number of the element. Also reduced their melting and boiling points. Alkali metals interact with many simple substances - oxidizing agents. In water reactions, they form soluble bases (alkali) soluble in water. Alkaline earth elements are the elements of the main subgroup of group II. Atoms of these elements contain two electrons in the external energy level. They are reducing agents, have a degree of oxidation of $ + $ 2. In this main subgroup are observed common laws in changing physical and chemical propertiesassociated with an increase in the size of atoms over the group from top to bottom, the chemical bond between atoms is also weakened. With an increase in the size of the ion, acidic and the main properties of oxides and hydroxides are enhanced. The main subgroup of the group III is elements of boron, aluminum, gallium, indium and tall lities. All items refer to $ P $ -lements. At the external energy level, they have three $ (S ^ 2p ^ 1) $ electron than the similarity of the properties is explained. The degree of oxidation is $ + $ 3. Inside the group with an increase in the charge of the kernel, metal properties increase. Bor - element-nonmetall, and aluminum has already metallic properties. All elements form oxides and hydroxides. Characteristics of transition elements ± copper, zinc, chromium, iron by their position in the periodic system of chemical elements D. I. Mendeleev and the peculiarities of the structure of their atomsMost metal elements are in the side groups of the periodic system. In the fourth period, the fourth electronic layer appears in potassium and calcium atoms, $ 4s $ -probill is filled, since it has less energy than $ 3D $ -prob. $ K, Ca - S $ -lements included in the main subgroups. Atoms from $ SC $ to $ zn $ is filled with $ 3D $ -probel electrons. Consider which forces act on an electron, which is added to an atom with an increase in the charge of the kernel. On the one hand, attraction atomic core, which causes the electron to occupy the lowest free energy level. On the other hand, repulsion already existing electrons. When the energy level is $ 8 $ electron (occupied by $ S- $ and $ R-$ orbital), their overall repulsive effect so hard that the next electron falls instead of an energy located in energy below $ D-$ orbital to a higher $ S- $ The orbital of the next level. Electronic structure of external energy levels in potassium $ ... 3D ^ (0) 4S ^ 1 $, calcium - $ ... 3D ^ (0) 4S ^ 2 $. The subsequent addition of another electron in scandadium leads to the beginning of the filling of $ 3D $ -rbital instead of even higher energy for energy $ 4r $ -rbitals. It turns out to be energetically more profitable. Filling $ 3D $ -Subitals ends at zinc having an electronic structure $ 1S ^ (2) 2S ^ (2) 2p ^ (6) 3S ^ (2) 3p ^ (6) 3D ^ (10) 4S ^ 2 $. It should be noted that the elements of copper and chromium observes the phenomenon of the "failure" of the electron. At the copper atom, the tenth $ d $ -Electron moves on the third $ 3D $ -prob. Electronic copper formula $ ... 3D ^ (10) 4S ^ 1 $. At the chromium atom at the fourth energy level ($ s $ -Orbital) there should be $ 2 $ electron. However, one of the two electrons moves to the third energy level, on an empty $ D $ -Rubital, its electronic formula $ ... 3D ^ (5) 4S ^ 1 $. Thus, in contrast to the elements of the main subgroups, where there is a gradual filling of the electrons of the atomic orbitals of the external level, the elements of side subgroups are filled with $ D $ -rbital the penultimate energy level. Hence the name: $ D $ -lements. Everything simple substancesFormed elements of subgroups of the periodic system are metals. Due to the larger number of atomic orbitals than in metal elements of the main subgroups, the atoms $ D $ -Elements form big number chemical ties among themselves and therefore create more durable crystal lattice. It is stronger and mechanically, and in relation to heating. Therefore, the metals of side subgroups are the most durable and refractory among all metals. It is known if the atom has more than three valence electrons, the element exhibits a variable valence. This provision refers to most $ D $ -Elements. The maximum valence, as the elements of the main subgroups, is equal to the number number (although there are exceptions). Elements C. equal number Valence electrons enter the group under one number $ (Fe, CO, Ni) $. In the $ D $ -Elements, the change in the properties of their oxides and hydroxides within the same period when moving from left to right, i.e. With an increase in their valence, it comes from the main properties through amphoteric to acid. For example, chrome has valency $ + 2, +3, + $ 6; And its oxides: $ Cro $ - the main, $ CR_ (2) O_3 $ - amphoterous, $ Cro_3 $ is acidic. The overall characteristics of non-metals of the main subgroups of IV ± VII groups due to their position in the periodic system of chemical elements D. I. Mendeleev and the characteristics of the structure of their atomsChemical elements - non-metalsThe very first scientific classification of chemical elements was dividing them to metals and non-metals. This classification has not lost its significance and now. Nemetalla- This is the chemical elements for atoms of which the ability to take electrons is characterized before the external layer is completed due to the existence, as a rule, on the outer electron layer of four and more electrons and the small radius of atoms compared to metals atoms. This definition leaves the elements of the VIII group of the main subgroup - inert, or noble, gases whose atoms have a complete outer electron layer. The electronic configuration of atoms of these elements is such that they cannot be attributed to any metals or non-metallam. They are those objects that share elements on metals and non-metals, occupying between them a border position. Inert, or noble, gases ("nobility" are expressed in inertia) sometimes refer to nonmetallam, but formally, in physical signs. These substances retain a gaseous state up to very low temperatures. So, helium does not switch to liquid state at $ t ° \u003d -268.9 ° C $. Inertia in chemical relations in these elements is relative. For xenon and crypton, the compounds with fluorine and oxygen are known: $ krf_2, xef_2, xef_4 and others. Undoubtedly, in the formation of these compounds, the inert gases performed as reducing agents. From the definition of non-metals it follows that the high values \u200b\u200bof electronegativity are characteristic of their atoms. It changes from $ 2 $ to $ 4 $. Non-metals are elements of the main subgroups, mainly $ p $ -Elements, the exception is hydrogen - S-element. All non-metal elements (except hydrogen) occupy in the periodic system of chemical elements D. I. Mendeleev the upper right corner, forming a triangle whose vertex is fluorine $ F $, and the base is a diagonal of $ B - AT $. However, it should be especially highlighted at the dual position of hydrogen in the periodic system: in the main subgroups I and VII groups. This is not by chance. On the one hand, a hydrogen atom, like alkali metal atoms, has one electron layer on the outer (and only) electronic layer ($ 1s ^ 1 $ electronic configuration), which is able to give, showing the properties of the reducing agent. In most of its compounds, hydrogen, like alkaline metals, shows the degree of oxidation of $ + 1 $. But the return of the electron at the hydrogen atom is harder than at alkali metal atoms. On the other hand, atom of hydrogen, as well as halogen atoms, before the end of the outer electron layer, there is a lack of one electron, so the hydrogen atom can take one electron, exhibiting the properties of the oxidant and characteristic of halogen the degree of oxidation - $ 1 $ in hydrides (compounds with metals like compounds Metals with halogens - halides). But the addition of one electron to the hydrogen atom occurs harder than halogen. Properties of atoms of elements - non-metalsOxidative properties prevail at atoms of non-metals, i.e. The ability to attach electrons. This ability characterizes the value of electronegability, which naturally changes in periods and subgroups. Fluoro is the strongest oxidizing agent, its atoms in chemical reactions Not able to give electrons, i.e. Show replacement properties. Configuration of the outer electronic layer. Other non-metals can exhibit replacement properties, although in a much weaker extent compared with metals; In periods and subgroups, their restorative ability varies in the reverse order compared with the oxidative.
Chemical elements-non-metals are only $ 16 $! Quite a bit, if you consider that $ 114 $ elements are known. Two non-metal elements are $ 76% of the mass of the earth's crust. This is oxygen ($ 49% $) and silicon ($ 27% $). The atmosphere contains $ 0.03% $ oxygen mass in earth Kore. Non-metals are $ 98.5% $ mass of plants, $ 97.6% $ human body weight. Non-metals $ C, H, O, N, S, R $ - organogen, which form the most important organic substances of the living cell: proteins, fats, carbohydrates, nucleic acids. In the air that we breathe are simple and sophisticated substancesAlso formed by non-metals (oxygen $ o_2 $, nitrogen $ n_2 $, carbon dioxide $ co_2, Water Couples $ n_2o $ et al.). Hydrogen is the main element of the universe. Many space objects (gas clouds, stars, including the sun) more than half consist of hydrogen. On the ground it, including the atmosphere, hydrosphere and a lithosphere, only $ 0.88% $. But this is by mass, and the atomic mass of hydrogen is very small. Therefore, the small content of it only seemingly, and from every $ 100 $ atoms on the ground $ 17 - hydrogen atoms. Explanatory note Thematic test "The patterns of changing the chemical properties of the elements and their compounds in periods and groups " Designed to prepare students for one State exam in chemistry. Target audience - grade 11. The wording of test tasks correspond to demonstration version Materials for Chemistry 2018. The tasks are compiled by analogy with tests published in the benefits "EGE. Chemistry: typical examination options: 30 options / ed. A.A. Cavery ", published in the publishing house" National Education "(Moscow, 2017) Patterns of changes in the chemical properties of elements and their compounds in periods and groups
Answer: From those specified in a number of chemical elements, select Three Elements, which in the periodic system of chemical elements D.I. Mendeleev are in the same group. Position the selected items in the order of increasing the acidic properties of their hydrogen compounds. From those specified in a number of chemical elements, select Three Elements, which in the periodic system of chemical elements D.I. Mendeleev are in the same group. Place the selected items in order to reduce their metal properties. From those specified in a number of chemical elements, select Three Elements, which in the periodic system of chemical elements D.I. Mendeleev are in one period. Position the selected items in the order of increasing the acidic properties of their higher hydroxides. From those specified in a number of chemical elements, select Three Elements, which in the periodic system of chemical elements D.I. Mendeleev are in one period. Place the selected items in the order of increasing the number of external electrons in the atoms of these elements. From those specified in a number of chemical elements, select Three Elements, which in the periodic system of chemical elements D.I. Mendeleev are in one period. Place the selected items in the order of increasing the radius of their atoms. From those specified in a number of chemical elements, select Three Elements, which in the periodic system of chemical elements D.I. Mendeleev are in one period. Place the selected items in order to enhance the oxidative properties of their atoms. From those specified in a number of chemical elements, select Three Elements, which in the periodic system of chemical elements D.I. Mendeleev are in the same group. Place the selected items in the reinforcement of the main properties of the oxides formed by them. From those specified in a number of chemical elements, select three metal. Place the selected items in order to reduce reducing properties. From those specified in a number of chemical elements, select Three Elements, which in the periodic system of chemical elements D.I. Mendeleev are in the same group. Answers Question 1. Question 2. Question 3. The main pattern of this change is to increase the metallic nature of the elements as Z grows. Especially clearly, this pattern is manifested in III-VIIA subgroups. For metals I A-III A-subgroups, there is an increase in chemical activity. In elements of IVA - VIIA-subgroups, as Z increases, there is a weakening of the chemical activity of the elements. Elements of B-subgroups have a change in chemical activity more difficult. The theory of the periodic system N. Bohr and other scientists in the 20s were developed. Xx in. and is based on a real scheme for the formation of electronic configurations of atoms. According to this theory, as Z increases, the filling of electronic shells and suburbs in the atoms of elements included in the periodic periodic periods occurs in the following sequence: Rooms periods Based on the theory of the periodic system, you can give the following period definition: The period is a set of elements starting the element with the value n. Equal to the period number, and l \u003d 0 (s-elements) and an ending element with the same value n and L \u003d 1 (P-elements) (see atom). The exception is the first period containing only 1S elements. Of the theory of the periodic system, the numbers of elements in the periods are followed: 2, 8, 8. 18, 18, 32 ... In the figure, the characters of each type of elements (S-, R-, D- and F-elements) are depicted on a specific color background: S-elements - on red, P-elements - on orange, D-elements - on blue, F-elements - On green. Each cell contains ordinal numbers and atomic weights of elements, as well as electronic configurations of external electron shells, which are mainly determined by the chemical properties of the elements. From the theory of the periodic system, it follows that elements with and equal to the period, and L \u003d 0 and 1. The elements are belonging to b-subgroups, the elements in the atoms of which are the completed shells that remained unfinished. That is why the first, second and third periods do not contain elements of B-subgroups. Structure of the periodic system of chemical elementsclosely related to the structure of atoms of chemical elements. As Z grows, there are periodically repetitive types of configuration of external electronic shells. Namely they define the main features of chemical behavior of elements. These features appear differently for elements of A-subgroups (S- and P-elements), for elements of B-subgroups (transient D-elements) and elements of F-families - lanthanides and actinoids. A special case represent elements of the first period - hydrogen and helium. For hydrogen, high chemical activity is characterized, because its only B-electron is easily cleaved. At the same time, the helium configuration (1st) is very stable, which causes its complete chemical inactivity. The elements of A-subgroups are filling out external electron shells (with N, equal to the period of the period); Therefore, the properties of these elements change significantly as Z groves. So, in the second period of lithium (2S configuration) - an active metal, an easily losing only valence electron; Beryllium (2S ~) is also metal, but less active due to the fact that its external electrons are more firmly connected to the nucleus. Further, the boron (2z "P) has a weakly pronounced metallic character, and all subsequent elements of the second period, which occurs in the construction of the 2r-submarine, are already non-metals. The eight-electronic configuration of the external electron shell of neon (2S ~ r ~) - inert gas - very durable. Chemical properties of the elements of the second period They are explained by the desire of their atoms to acquire the electronic configuration of the nearest inert gas (helium configuration - for elements from lithium to carbon or configuration of neon - for elements from carbon to fluorine). That is why, for example, oxygen cannot exercise the highest oxidation equal to the number of the Group: because it is easier for it to achieve neon configuration by purchasing additional electrons. The same nature of the change in properties is manifested in the elements of the third period and in the S and P-elements of all subsequent periods. At the same time, the weakening of the strength of the connection of external electrons with the nucleus in A-subgroups as Z grows appears in the properties of the corresponding elements. Thus, for S-elements there is a noticeable increase in chemical activity as Z groves, and for P-elements - the increase in metal properties. In the atoms of transitional D-elements, the shells not completed earlier with the value of the main quantum number and, per unit of the smaller period of the period. For individual exceptions, the configuration of the external electronic shells of atoms of transition elements is NS. Therefore, all D-elements are metals, and that is why changes in the properties of the 1-elements as Z grows are not so cutting, as we have seen in S and P-elements. In the highest degrees of oxidation, D-elements show a certain similarity with the p-elements of the corresponding groups of the periodic system. The features of the properties of triad elements (VIII B-subgroup) are explained by the fact that the D-submarine is close to completion. That is why iron, cobalt, nickel and platinum metals are usually not inclined to compound the highest degrees of oxidation. The exceptions are only ruthenium and osmium, giving oxides RUO4 and OSO4. The elements of the I- and II B-subgroups D-submarine actually turns out to be completed. Therefore, they exhibit the degrees of oxidation equal to the group number. In the atoms of lanthanides and actinoids (all of them metals) there are completed previously not completed electronic shells with the value of the main quantum number and two units less than the period number. In atoms of these elements, the configuration of the external electron shell (NS2) is saved unchanged. At the same time, F-electrons actually do not affect chemical properties. That is why Lantanoids are so similar. Aktinoids the situation is much more complicated. In the zero charge intervals, Z \u003d 90 - 95 electrons BD and 5 can take part in chemical interactions. And hence it follows that actinoids exhibit a much wider range of oxidation degrees. For example, for neptural, plutonium and americium are known compounds where these elements act in seven valence state. Only in the elements, starting from Curia (z \u003d \u003d 96), becomes a steady trivalent state. Thus, the properties of actinoids differ significantly from the properties of lanthanides, and both families can therefore be considered similar. The Aquinide family ends with Z \u003d 103 (Laurerenis). Assessment of the chemical properties of Kurchatyia (Z \u003d 104) and Nielsboria (Z \u003d 105) shows that these elements must be analogues of respectively hafnia and tantalum. Therefore, scientists believe that after the Aquidide family in atoms, the systematic filling of the 6D submarine begins. The finite number of elements that covers the periodic system is unknown. The problem of its upper border is, perhaps, the main mystery of the periodic system. The most difficult element that managed to detect in nature is plutonium (z \u003d 94). The achieved limit of artificial nuclear synthesis is an element with a sequence number 107. The question remains open: will it be able to get elements with large sequence numbers, what and how much? It is impossible to answer it for now. 1. What is the computer science? computer techologies information is intangible handle. smell sound speech man taste photos encryption information transfer data storage sort list database search 6. What is coding? information search tool distortion of information changing the type of information Test on the topic: "Information and information processes" 1. What is the computer science? any processes and phenomena associated with information programming for computers the relationship of phenomena in nature computer techologies mathematical methods for solving problems 2. Mark all the right statements. information is intangible information is the reflection of the real world. information characterizes a variety when receiving information, knowledge uncertainty decreases there is a strict definition of information 3. Check the types of information that the computer does not know how handle. smell sound speech man taste photos 4. Select processes that can be called information processing. encryption information transfer data storage sort list database search 5. Mark all the right statements. information can only exist with the carrier information storage is one of the information processes. in order to extract information from the message, a person uses knowledge information processing is a change in its content. when writing information, media properties change 6. What is coding? information search tool recording information in another system of signs distortion of information changing the type of information changing the number of information choosing the desired elements change order elements removing unnecessary elements to transfer information? principles? _______________________________________________________________ solving some tasks? _______________________________________________________________ yourself? _______________________________________________________________ systems? _______________________________________________________________ 7. What phrase can serve as a definition of sorting? choosing the desired elements arrangement of the list elements in a given order alphabetic lines alphabet change order elements removing unnecessary elements 8. What is the name of the change in the properties of the carrier, which is used to transfer information? _______________________________________________________________ 9. What are the knowledge of the knowledge that are facts, laws, principles? _______________________________________________________________ 10. What are the knowledge that are algorithms solving some tasks? _______________________________________________________________ 11. How to call a person's ideas about nature, society and the most yourself? _______________________________________________________________ 12. Mark all the right statements. the information obtained depends on the knowledge of the recipient the information obtained depends only on the received message. obtaining information always increases knowledge knowledge increases only when the information received is partially known the same information can be presented in different forms. 13. How to call information fixed (encoded) in some form, in particular, in computer information systems? _______________________________________________________________ Answer: 1 2 3 4 5 6 7 a, b, g a B C D a, G. a, g, d a, B, D b, G. 8 9 10 11 12 13 signal delarative procedural knowledge a, g, d |
Popular:
New
- Tag: functions of several variables Geometric meaning of the differential of two variables
- Theorem on the change in the number of movement of the dynamics of the theorem on the change in the amount of movement
- Changing the amount of mechanical system of the dynamics of the theorem on the change in the amount of movement
- Speed \u200b\u200bof free fall
- How to calculate the limits of functions without using differential calculus
- How to find a gradient function
- Distance from point to point: Formulas, examples, solutions
- Solution of all types of limits with a detailed decision
- Cross-section inertia moments
- Determining the distance between the point and the plane, direct and plane, between planes and cross-lived straight